Alfa Neuseal
Alfa NeuSeal
An innovative tissue adhesive for various derma applications.
- Product type: Medical Device of Class IIa.
- Registration: Asia, India, Africa, and EU (in progress).
- Application: Dermatology – Tissue Adhesive.
- Dosage form: Sterile tissue Adhesive (Sterile Blue color solution).
- Recommended dosage: As required (available as 0.25 ml or 0.5 ml).
- Manufacturing counties: India.

1. Overview of the advantages of Alfa NeuSeal
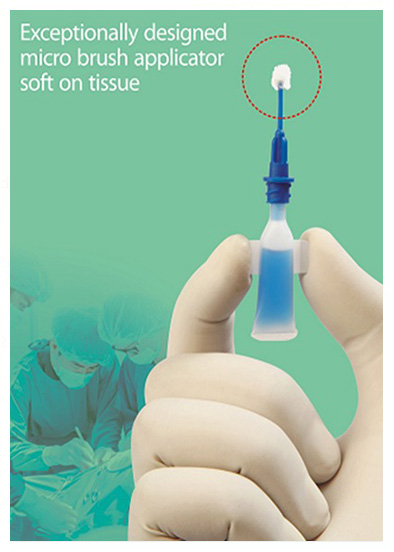
- Exceptionally designed micro brush applicator soft on tissue.
- Adhesive conveniently can be stored at room temperature.
- Airtight Aluminum pack protest from moisture.
- Adhesive is completely biodegradable.
- Effectively used on everything from wound, cuts to incision.
- Forms a microbial barrier.
2. Product application areas
- Laparoscopic surgeries
- Fundal & Esophageal varices
- General surgery
- Paediatrics applications
- Neurosurgery
- Ophthalmic
3. Composition – Synergistic ingredients
4. Clinical Evidence
5. Mechanism of Action
6. Patents
7. Technical Data
8. Product Background
- Cyanoacrylates were synthesized in the 1940s and were first used for wound closure in 1959. The early short-chain tissue adhesives, methyl-2 and ethyl-2-cyanoacrylate, while effective, had limited use because of rapid degradation into cyanoacetate and formaldehyde, which caused significant tissue toxicity resulting in acute and chronic inflammation.
- Longer-chain cyanoacrylates, n-butyl-cyanoacrylate, and 2-octylcyanoacrylate, degrade slowly, limiting the accumulation of toxic by-products in tissues, making them safe for topical skin closure.
- In 1998, the US FDA gave approval for use of the first topical skin adhesive.
- Since that time, several new skin adhesives have entered the market. presently, there are two types of topical skin adhesives being used: n-butylcyanoacrylate and 2-octylcyanoacrylate. both of these topical skin adhesives are FDA-approved.
US FDA classification of topical skin adhesives
- The FDA has three established categories (classes) of devices, depending on the regulatory controls needed to provide reasonable assurance of their safety and effectiveness; these categories are class i (general controls), class ii (special controls), 11 and class iii (premarket approval).
- In 2008, the fda reclassified topical adhesives as class ii rather than class iii.
- Class i skin adhesives are available over the counter (OTC) in drug stores.
- The FDA designates devices as class ii if general controls by themselves are insufficient to provide reasonable assurance of safety and effectiveness, but there is sufficient information to establish special controls to provide reasonable assurance of the safety and effectiveness of the device for its intended use.
- After reviewing the relevant information, the fda determined that “tissue adhesive with adjunct wound closure device intended for topical approximation of skin” can be classified into class ii with the establishment of special controls in order to provide reasonable assurance of the safety and effectiveness of the device
